Abstract
BiTE ® (Bispecific T-cell Engager) constructs represent a novel immunotherapeutic strategy that recruits T cells against cancer cells independent of their TCR specificity. Currently, two CD33xCD3 BiTE ® antibody constructs (AMG 330 & AMG 673) are being investigated in phase I dose escalation trials in patients with relapsed/refractory Acute Myeloid Leukemia (AML) with early evidence of acceptable safety and anti-leukemic activity (Ravandi et al., ASH 2020; Subklewe et al., EHA 2020). So far, details of BiTE ® mediated T-cell engagement and information on parameters contributing to their efficacy need more investigation. Therefore, we aimed to characterize the interplay between target and effector cells to deepen our mechanistic understanding of BiTE ® construct mediated T-cell engagement.
Previously, we have created a novel in vitro model system with murine Ba/F3 cells expressing human (hu) CD33 ± huCD80 ± huCD86 ± huPD-L1 to study T-cell proliferation and cytotoxicity induced by AMG 330. Using that system, we showed that expression of T-cell co-signaling receptors on target cells modulate AMG 330 induced T-cell activity (Marcinek et al., ASH 2018, EHA 2019). Here, we hypothesize that expression of costimulatory molecules impacts BiTE ® mediated immune synapse formation and consecutive downstream signaling in BiTE ® construct activated T cells.
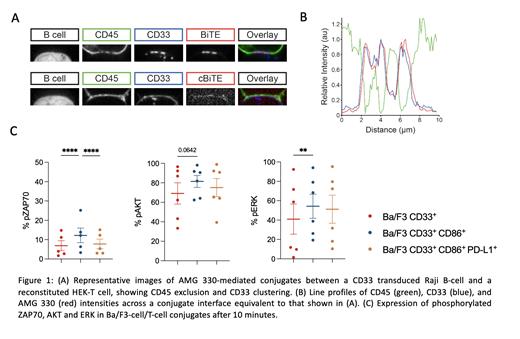
To study whether AMG 330 can induce synapse formation and TCR triggering we used a previously described reconstituted T-cell system, which consists of non-immune (HEK) cells introduced with genes encoding the TCR and other proteins (e.g. CD45) required for the regulation of TCR phosphorylation (James et al., Nature 2012). HEK-T cells were incubated with huCD33 transduced RajiB cells in presence of fluorescently labeled AMG 330 or a control BiTE® (cBiTE) construct to allow cell conjugation. A spinning disc confocal microscope system was used to image cells. To pinpoint the role of T-cell co-signaling receptors in immune synapse formation we incubated differentBa/F3 cell constructs or primary AML (pAML) cells with healthy donor T cells in the presence of AMG 330 and analyzed intensity of LFA-1 expression within the synapse using an Imaging Flow Cytometer. Furthermore, we determined phosphorylation of ZAP70, AKT and ERK in conjugated T cells after various time points by phosphoflow cytometry.
We observed that AMG 330, in contrast to cBiTE®, induced TCR triggering reflected by exclusion of CD45 from the RajiB-T-cell-interface. Simultaneously clustering of CD33 occurred in AMG 330 induced cell-cell-interfaces (Fig. 1A/B). The percentage of conjugates formed with huCD33 + Ba/F3 cells was significantly higher in constructs expressing huCD86, compared to those expressing no costimulatory antigens or additional huPD-L1 (Mean % in huCD33 + Ba/F3: 2.8 vs. huCD33 + CD86 +.Ba/F3: 4.2 [p=0.0031] vs. huCD33 + huCD86 + PD-L1 + Ba/F3: 2.8 [p=0.0018]). This was accompanied by LFA-1 accumulation within the T-cell-Ba/F3 cell synapse (Mean of MFI in huCD33 + CD86 +.Ba/F3: 10,933 > huCD33 + huCD86 + PD-L1 + Ba/F3: 7,749 > huCD33 + Ba/F3: 7,028). For downstream signaling in T cells after engagement with Ba/F3 cell constructs in the presence of AMG 330, we observed that kinase phosphorylation was highest after 10 minutes in CD86 co-expressing Ba/F3 cells (Mean % of phosphorylation in T-cell conjugates with huCD33 + vs huCD33 + huCD86 + vs huCD33 + CD86 +.PD-L1 + Ba/F3: pERK 40.9 vs 54.3 [p=0.0064] vs 51.2 %; pAKT: 69.1 vs 81.5 [p=0.0642] vs 75.1 %; pZAP70: 6.9 vs 12.2 [p<0.0001] vs 7.7 % [p<0.0001]) (Fig. 1C).
Finally, we evaluated if these finding could also be observed in pAML samples. For that, we determined LFA-1 expression intensity within AMG 330-induced pAML-T-cell synapses. We used CD33 + pAML samples with either high CD86 and no PD-L1 expression or vice versa. Comparing synapse formation between these samples, LFA-1 intensity was 4.6-fold higher in the CD86 + PD-L1 - sample compared to the CD86 - PD-L1 + pAML.
Taken together, our data unravel molecular mechanisms of BiTE® construct induced immune synapse formation, highlighting the role of costimulatory molecules in this process. They support the notion that T cell co-signaling receptors like CD86 and PD-L1 modulate T-cell response in an early event manner. Prospective analyses in clinical trials are needed to validate the relevance of checkpoint molecule expression on target cells as a potential predictive biomarker for response.
Brauchle: Adivo: Current Employment. Lacher: Roche: Research Funding. Kischel: Amgen GmbH Munich: Current Employment. von Bergwelt: Roche: Honoraria, Research Funding, Speakers Bureau; Miltenyi: Honoraria, Research Funding, Speakers Bureau; Mologen: Honoraria, Research Funding, Speakers Bureau; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Novartis: Honoraria, Research Funding, Speakers Bureau; Astellas: Honoraria, Research Funding, Speakers Bureau; MSD Sharpe & Dohme: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Research Funding, Speakers Bureau. Theurich: Amgen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Buecklein: Novartis: Consultancy, Other: congress and travel support, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Miltenyi: Research Funding; Kite/Gilead: Consultancy, Honoraria, Other: Congress and travel support, Research Funding; BMS/Celgene: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. Subklewe: Janssen: Consultancy; Seattle Genetics: Consultancy, Research Funding; Roche: Research Funding; Novartis: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Speakers Bureau; Klinikum der Universität München: Current Employment; Takeda: Speakers Bureau; MorphoSys: Research Funding; Miltenyi: Research Funding; Gilead: Consultancy, Research Funding, Speakers Bureau; Amgen: Consultancy, Research Funding, Speakers Bureau; BMS/Celgene: Consultancy, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal